Pancreatic Cancer
The best minds of medicine today have no solution for pancreatic cancer. From your physician’s perspective, pancreatic cancer is an incurable disease. Prognosis is terminal. Accordingly, all treatments are only intended to extend life or provide palliative care. Surgery, radiation and chemotherapy have minimal impact and may provide only minimal improvement to survival. Alternatively, research supports that treatments may actually accelerate the progression of the disease. Yet, since the cancer is terminal with no cure, physicians, oncologists, are provided the latitude to experiment. Sequential chemotherapy drugs are offered to “try” if the previous drug did not work (created a resistant, more aggressive cancer). Since the patient is considered to be terminal, long-term, debilitating side effects of treatments are not considered. Usually diagnosed in late-stage disease phase, the cancer is typically already metastasized to other organs.
Nutritional agents have demonstrated “spontaneous cures” for pancreatic cancer. New formulation technologies such as nanoparticle encapsulation have increased the bioavailability of these agents by factors of 50x to over 90x. By targeting multiple disease pathways at the cell level, progression of pancreatic cancer can be stopped, tumors reduced. This can be achieved over a short period of time, typically 90 days, without side effects, without the cancer becoming resistant to treatment it is with chemotherapy drugs.
“Pancreatic cancer is known to be one of the most lethal cancers. The majority of patients present with advanced stage disease, making curative approach unachievable. In untreated patients, the median survival does not exceed 6 months in metastatic disease and 10 months in locally advanced disease. Furthermore, the 5-year survival rate remains poor even in patients with early stage disease who are surgical candidates. The detrimental outcome is related to the high potency of developing metastasis which can be detected at diagnosis, when the disease progresses or relapses after surgery. Although the liver is the most common site of pancreatic cancer metastases, the cancer can escape the liver in some cases and metastasize to the lung or other distant organs.”
Pulmonary metastases in pancreatic cancer, is there a survival influence?
Ayham Deeb, Sulsal-Ul Haque, and Olugbenga Olowokure
Journal of Gastrointestinal Oncology. 2015 Jun
\metastasis survival pancreatic 2015.pdf
“Gemcitabine treatment, the current gold standard therapeutic agent (chemotherapy) of pancreatic cancer also has been shown to induce cachexia in an experimental model of pancreatic cancer [11], similar to other anti-cancer agents such as taxanes [12]. Considering all these facts, there is an instant need of alternative therapeutic agents that possess anti-cancerous as well as anti-cachectic properties.”
“Our results demonstrate that (natural agent) silibinin inhibits pancreatic cancer cell growth in a dose-dependent manner and reduces glycolytic activity of cancer cells. Our LC-MS/MS based metabolomics data demonstrates that silibinin treatment induces global metabolic reprogramming in pancreatic cancer cells.”
“We observed a significant reduction in tumor growth rate and tumor volume upon necropsy in silibinintreated tumor-bearing mice in comparison to the control group (Figure 5A–5C). We also observed a significant difference between body weight of the control group and silibinin-treated mice (Figure 5D), with significantly reduced weight loss in the silibinin-treated tumor-bearing mice. Furthermore, we observed reduced tumor cell proliferation in the silibinin-treated group in comparison to the control group, as evident by reduced Ki67-positive cells in the silibinin-treated tumor sections (Figure 5E). We also observed reduced tumoral c-MYC, GLUT1, and pSTAT3 expression in silibinin-treated mice in comparison to the controls (Figure 5E), which corroborates our results from cell culture-based studies.”
Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth
Surendra K. Shukla1, Aneesha Dasgupta1,2, Kamiya Mehla1, Venugopal Gunda1, Enza Vernucci1, Joshua Souchek1, Gennifer Goode1, Ryan King1, Anusha Mishra1, Ibha Rai1, Sangeetha Nagarajan1, Nina V. Chaika1, Fang Yu3, Pankaj K. Singh1,2,4,5
1 The Eppley Institute for Research in Cancer and Allied Diseases,
2Department of Biochemistry and Molecular Biology,
3Department of Biostatistics,
4Department of Pathology and Microbiology,
5Department of Genetics Cell Biology and Anatomy,
University of Nebraska Medical Center, Omaha, Nebraska 68198, USA
Oncotarget, Vol 6, No. 38, 2015
\silibinin metab apop pancreatic 2015.pdf
“As a result, satisfactory therapeutic effects were obtained, such as improvements in biological functions and spontaneous cure power.”
“CT images showed marked reductions of the pancreatic cancer and liver metastases (figure 1b).”
“… three months after treatment initiation, the tumor markers rapidly decreased (CEA 93.6 ng/ml from 441 ng/ml, CAI9-9 6,300 U/ml from 61,000 U/ml)…”
“The diameter of the pancreatic cancer was almost unmeasurable, and the liver metastases reduced in the same way… Early cancer on the anterior wall of the angular lesser curvature stomach completely disappeared (figure 3b, arrow). A biopsy revealed no malignant tumor cells.”
“The patient had the decreased sugar metabolism peculiar to pancreatic cancer, but the combination therapy allowed easy control of the blood sugar, and a good nutritional state was maintained.”
“This therapy is one treatment option that prolongs the lives of patients with terminal cancer while preserving physical strength and maintaining QOL.”
A Case Where an Immunomodulatory Food was Effective in Conservation
Therapy for Progressive Terminal Pancreatic Cancer
K Kakatani, Hanzomon Gastrointestinal Clinic
Clinical Pharmacology and Therapy, Vol 14, No. 3, May 2004
\MGN-3 spontaneous pancreatic 2004.pdf
“Patients received 8 g curcumin by mouth daily until disease progression, with restaging every 2 months. Serum cytokine levels for interleukin (IL)-6, IL-8, IL-10, and IL-1 receptor antagonists and peripheral blood mononuclear cell expression of NF-nB and cyclooxygenase-2 were monitored.”
“Patients took oral curcumin daily for 8 weeks. The starting dose was 8 g/d. The patients could not receive any concomitant chemotherapy or radiotherapy, although they could receive supportive care. Patients who had stable disease or better after 8 weeks received continued therapy with curcumin at the same dose and schedule.”
“There was no dose-limiting toxicity; dosing was limited by the number of pills that patients could or would swallow daily.”
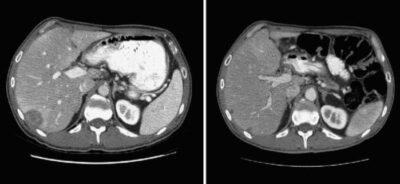
“Fig. 2. Computed tomography scan of the abdomen showing hepatic lesions in patient [8]. The computed tomography scans on the left were done pre-therapy; the one on the right were done at 2 mo after starting curcumin. There was an overall 73% decrease in the size of liver lesions by Response Evaluation Criteria in Solid Tumors.”
Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer
Navneet Dhillon,1Bharat B. Aggarwal,2 Robert A. Newman,2 Robert A.Wolff,3 Ajaikumar B. Kunnumakkara,2
JamesL . Abbruzzese,3 Chaan S. Ng,4 Vladimir Badmaev,5 and Razelle Kurzrock1
1Phase I Program, Department of Investigational Cancer Therapeutics, 2Department of Experimental Therapeutics, 3Department of Gastrointestinal Medical Oncology, Division of Cancer Medicine, and 4Department of Diagnostic Radiology,The University of Texas M. D. Anderson Cancer Center, Houston,Texas and 5Sabinsa Corporation, Piscataway, NewJersey
2008 American Association for Cancer Research.
\curcumin clin trial pancreatic 2008.pdf
“Continuous exposure of pancreatic cancer cells to dietary bioactive agents does not induce drug resistance unlike chemotherapy”
“Several laboratory and animal studies exist and suggest that sulforaphane and quercetin inhibit proliferation and metastasis and enhance apoptosis and eliminate CSC features in pancreatic cancer.”
“Quercetin and sulforaphane selectively reduce the viability in malignant cells.”
“Continuous quercetin and sulforaphane exposure reduces tumorigenicity in vivo.”
“Continuous quercetin and sulforaphane exposure reduces the expression of progression markers.”
Continuous exposure of pancreatic cancer cells to dietary bioactive
agents does not induce drug resistance unlike chemotherapy
P Fan, et al., Molecular OncoSurgery, University of Heidelberg,
Cell Death and Disease, 2016
\bioactive agents pancreatic 2016.pdf
“A growing body of evidence supports the idea that curcumin is a promising anticancer drug. Curcumin has anticancer effects, both alone and in combination with other anticancer drugs, through the modulation of a variety of molecular targets in preclinical models.”
“Curcumin can modulate the activity of a variety of molecules that play important roles in cancer progression, with more than 30 molecular targets identified to date[38].”
“… curcumin can downregulate the expression of miR-21[28], which is overexpressed in a variety of tumors, including pancreatic cancer, and is considered to be an oncogenic miRNA[41].”
“Several phaseⅠand pharmacokinetic studies have been conducted using curcumin, and they found no dose-limiting toxicity (DLT) up to at least 12 g/d when administered orally to both healthy volunteers[42,43] and cancer patients[44-46].”
“Furthermore, curcumin treatment was found to be safe in patients with pancreatic cancer, and no toxicity was associated with curcumin intake.”
“In total, 21 patients who showed disease progression during previous gemcitabine-based chemotherapy were enrolled in the study. The addition of an 8-g daily oral curcumin (to gemcitabine chemo) dose did not increase the risk of clinically relevant toxicity, and the toxicity profile of the combined drugs was comparable with that observed in pancreatic cancer patients treated with gemcitabine-based chemotherapy alone. Cumulative toxicity from curcumin was not observed, and 4 patients were able to continue this intake regimen for over 6 mo, indicating that this agent is safe for long-term use.”
